In valve regulated batteries the connecting strap and the plate lugs (top lead) are covered by a thin liquid film of H2SO4 solution. H2SO4 is consumed by the formation of a PbSO4 corrosion layer as a result of which the strap loses it’s cathodic protection. Often the life of the battery is limited by corrosion of the negative semi-block top lead. In battery manufacturing practice, Pb-Sn alloys are used for production of straps. LABD team has examined creeping of the thin liquid film up a strap electrode, the structure and phase composition of the corrosion layer formed on the electrode surface, as well as the rate of corrosion of Pb-Sn and Pb-Sn-Se alloys
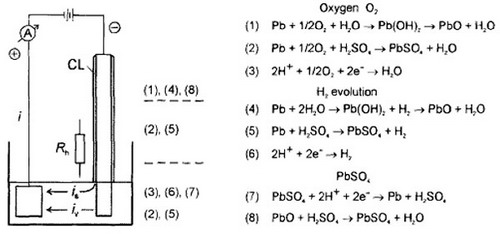
Corrosion layer formed on the top lead. Special electrodes and experimental setup have been designed to determine corrosion layer properties. The distribution of potential along the height of the strap electrode ahs been measured. It has been found that the corrosion layer comprises tree sublayers: (i) outer porous layer, built of small PbSO4 crystals; (ii) sublayer built of large PbSO4 crystal formed as a result of recrystallization of the small crystals; (iii) inner layer featuring small particles and liquid film covering the metal surface. The formed corrosion layer can be divided in three zones along the strap height: (i) strap zone immersed in the solution and up to 1 cm above the solution level, and controlled by the applied external potential; (ii) PbSO4 zone (at 2-4 cm strap height); (iii) amorphous zone above 4 cm (probably lead oxide). The distribution of the parallel electrochemical reactions that proceed along the top lead has been determined.
Corrosion of Pb-Sn and Pb-Sn-Se alloys. Since the most widely used alloy in top lead manufacture is Pb-Sn, the impact of Na2SO4 and Se on the corrosion rate of this alloy has been evaluated. Introduction of Na2SO4 to the H2SO4 solution leads to a sevenfold increase in corrosion rate of Pb-Sn alloy, whereas addition of 0.03% Se to the alloy suppresses completely this effect of Na2SO4. When the Sn content in the alloy is below 1%, the rate of corrosion increases. Selenium suppresses this effect, too, in alloys containing 0.6% Sn. Selenium has an anticorrosion effect and acts as a grain refiner in Pb-Sn alloys decreasing also the size of PbSO4 crystals and facilitating their nucleation.
References
- D. Pavlov, M. Dimitrov, G. Petkova, H. Giess, C. Gnehm, Corrosion of strap and lugs of the negative plates in VRLA batteries, Extended Abstracts, Fall Meeting of the Electrochemical Society inc., Miami Beach, Florida, Vol. 84-2,p.254-255, Pennington NJ, USA, 1994
- D. Pavlov, M. Dimitrov, G. Petkova, H. Giess, C. Gnehm, The effect of selenium on the electrochemical behaviour and corrosion of Pb-Sn alloys used in lead-acid batteries, J. Electrochem. Soc., 142 (1995) 2919
Keywords: LA battery strap, top lead, Pb-Sn alloys, corrosion of Pb-Se alloys |